Laboratory Good Manufacturing Practices (GMP)
To achieve safety and efficacy, manufacturing cell products for therapeutic purposes requires rigorous standards to regulate the premises, procedures, processes, final product, and personnel. GMP laboratories follow and strictly adhere to GMP standards and regulations to avoid health hazards, reduce errors in manufacturing, ensure proper documentation, and minimize variances in final product.
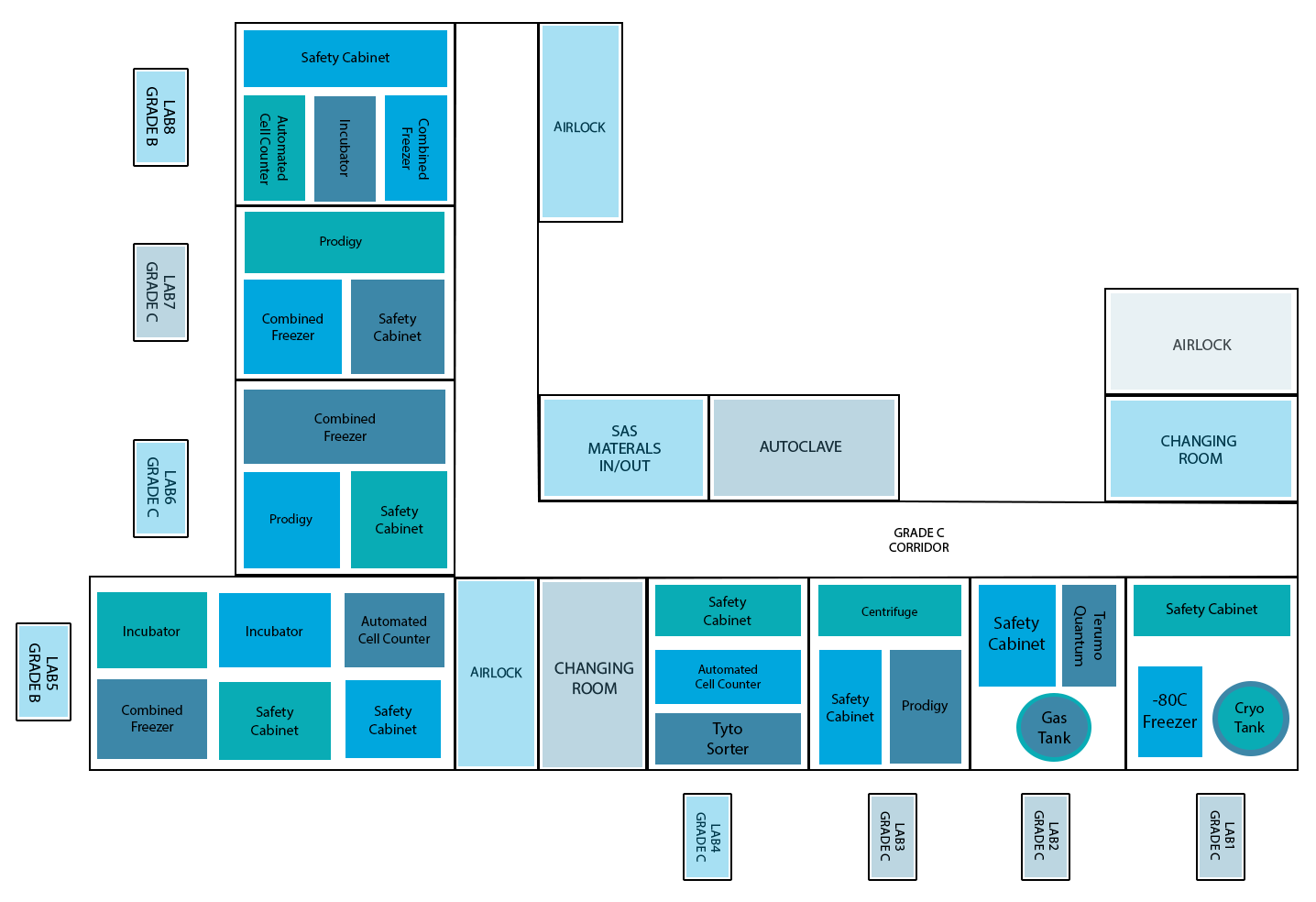
To achieve safety and efficacy, manufacturing cell products for therapeutic purposes requires rigorous standards to regulate the premises, procedures, processes, final product, and personnel. GMP laboratories follow and strictly adhere to GMP standards and regulations to avoid health hazards, reduce errors in manufacturing, ensure proper documentation, and minimize variances in final product. GMP labs can be graded from A (strictest) to D (least strict) depended on the degree of cleanliness and sterility. ADSCC’s GMP lab includes 2 B-type suites, and 6 C-type suites. In both suites, sinks and drains are prohibited, and the number of airborne particles is limited. The main difference between B and C-type suites are the physical parameters such as air changes, air filtration, room pressurization, and air velocity. B grade suites require higher degree of air changes, air filtration, room pressurization, and air velocity. Also, the maximum number of airborne particles is limited to 3520 x 104 μm/m3 . In ADSCC, grade B labs will be limited to cell culturing applications, and grade C for closed system cell isolation and expansion. The layout of the lab is designed for filtered air flow, and GMP- compliant equipment are designed to have a small footprint to avoid impacting air quality and facility design. Each instrument with its own application must be placed in a separate room to reduce the risk of cross contamination.